Order of a Reaction
Order of a Reaction: Overview
This topic covers the concept of Order of a Reaction.
Important Questions on Order of a Reaction
For the reaction , . The reaction is of :
For a reaction, in an aqueous medium, the rate of reaction is given by
The role of is.
For the following elementary reaction, determine its order of reaction and the dimensions of the rate constant:
For a reaction, , the rate is given by , hence, the order of the reaction is:
For the reaction product
order of reaction is :-
Statement : The exponents for concentration do not necessarily match the stoichiometry coefficients in the rate law expression.
Statement : In the rate law expression, exponents for concentration are determined by experiments not by balanced chemical reaction.
Find out the order for the given reaction
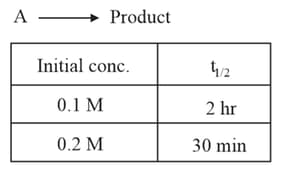
Radioactive decay is an example of which order of reaction?
Calculate the overall order of the reaction, given that a reaction involving A, B and C as reactants is found to obey the rate law, rate=. When the concentration of A, B and C are doubled separately, the rate is also found to increase two, zero and four times respectively.
Suppose in a chemical reaction , it is found that the rate of the reaction doubles when the concentration of is increased four times. The order of the reaction with respect to is
Which principle of Scientific Management involves studying the time taken to perform a task?
Which of the following is a measure of employment levels?
What is the rate of interest called that is specified on the face of the debenture certificate?
Which of these is a principle of scientific management proposed by Frederick Taylor?
What is checked during the comparison step in the controlling process?
Which of the following involves setting benchmarks for performance in the controlling process?
Which of the following is the first step in the process of controlling?
In what order are the transactions recorded in the Receipt and Payment account?
What is the first step in the controlling process?
